ENGEL in medical technology: the e-motion 220 impresses
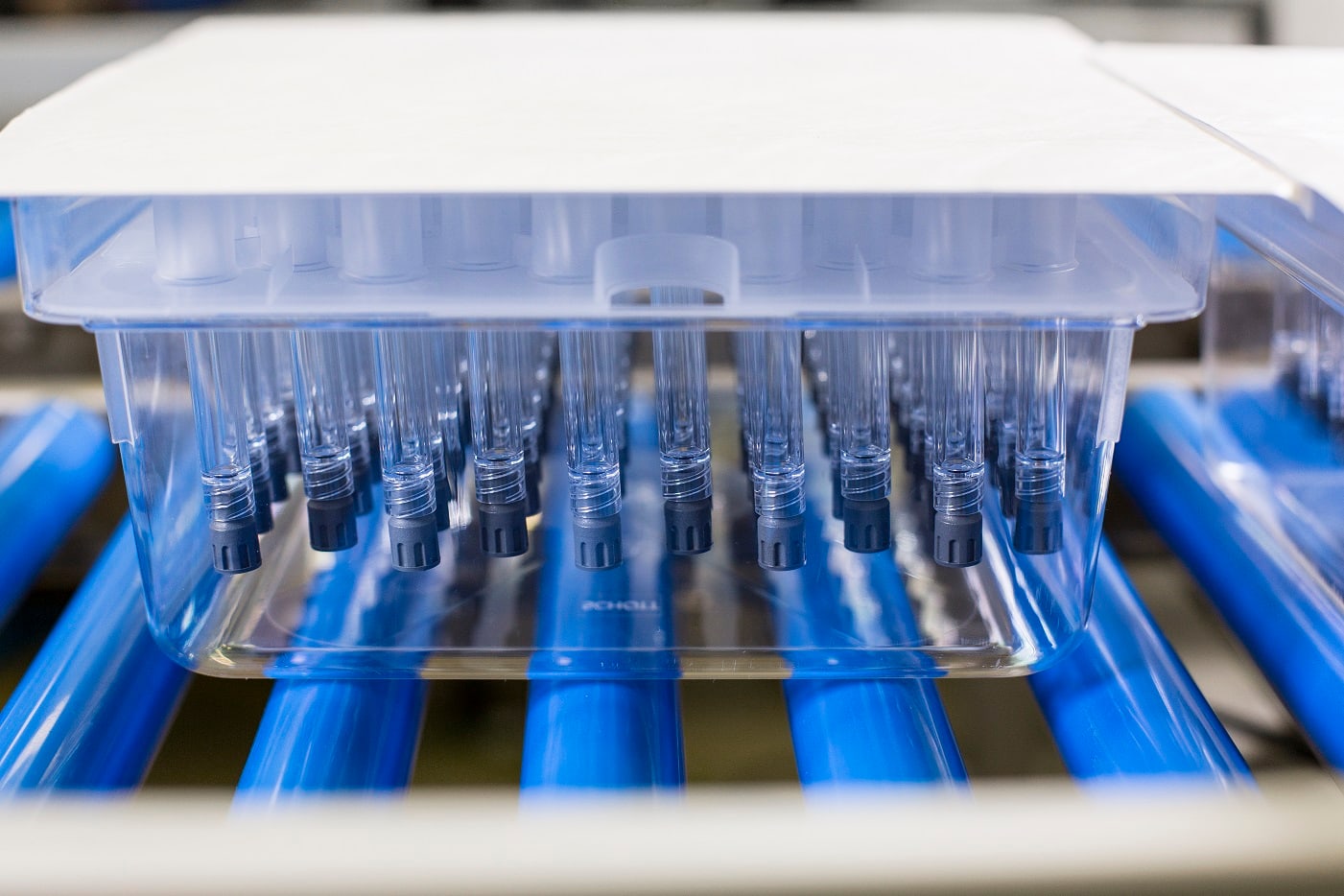
The Schott Group produces prefillable syringes made of COC (cyclo olefin copolymer) on an ENGEL e-motion 220 for the Pharmaceutical Systems business unit at the St. Gallen Competence Center. These syringes require maximum precision, cleanliness and performance in production.
One injection moulding process – many benefits
In addition to glass syringes, the Schott Group has also been manufacturing plastic syringes for almost 20 years. The plastic syringes are mostly made of polypropylene (PP), a relatively small proportion of COC or COP (cyclo olefin polymer). “We serve a niche market in the 'prefillable syringes' segment,” says Tom Van Ginneken, Global Product Manager at Schott, explaining the situation. The fairly small market for prefillable syringes made of COC can be attributed to how well glass is established. But there are various reasons that prompt a pharmacist to opt for a COC product despite this: the material is inert, bio-compatible and exhibits low protein absorption. Compared with PP, COC has 10 times higher barrier properties against both moisture and oxygen. The break resistance of plastic is another strength compared to glass. ”One advantage of COC in manufacturing is flexible design. The thread can be created with high precision using injection moulding technology. You can't do that with glass”, as Jens Meiss, a process engineer at Schott explains, pointing out another benefit of COC compared with glass.
Schott supplies ready-to-use, prefillable syringes to the customer. The customer can immediately fill the product in-house. In syringes made of COC, a drug remains stable for 3 years and retains its effectiveness, thanks to the excellent barrier properties. This makes COC the material of choice, also for premium medication.
The step to an integrated solution
In addition to glass syringes, the Schott Group identified increased market potential due to stronger demand for prefillable plastic syringes. This raised the question of expanding production capacity and at the same time changing the generation of injection moulding machines. “This prompted us to look at the market to determine who could deliver the best integrated solution for our production. ENGEL made this easy for us. In 2015 we purchased the first system with an integrated robot and two years later, we are establishing a fully automated production line,” says Michael Feldhaus, Head of Sterile Technology explaining the situation. And he adds: “The modular design and thus the flexibility of the large machine convinced us. We can use screws from 40 to 60 mm and, thanks to a clamping force of 220 tons, cover the entire portfolio from small to large”.
The recently delivered injection moulding machine is a cleanroom version of the ENGEL e-motion 940/220 T including brand-name automation. All 32 high-transparency syringes, which are internally siliconised at the top, are removed at the same time and deposited separately. “The ENGEL machines are fundamentally designed to accommodate to customer requirements by adding options. The requirements in this case were maximum precision, cleanliness and efficiency, meaning short cycles. That's why we decided to offer the e-motion machine. As the movements here are all-electric, we can raise precision to a new level compared to the previous production. This guarantees maximum reproducibility. With a series of medical options – following the normal approach at ENGEL – we made the machine cleanroom capable. We also implemented a number of special options, such as taking in the mould laterally with a roller conveyor instead of a crane,” says Markus Schertler, Managing Director of ENGEL Switzerland, explaining the cleanroom configuration. GMP documentation by ENGEL provides proof that the strict requirements in medical technology – qualification and calibration – are met.
Tie-bar-less prototype machine
Schott started working with ENGEL injection moulding machines in 2015 when it purchased a tie-bar-less 80-ton machine, and e-victory 170/80 featuring an integrated ENGEL easix multi-axis robot. This is a system for sampling and small runs. “We produce prefillable COC syringes on the e-victory under the same process conditions as we have with our large-scale system, allowing us to expand our expertise and capabilities. Additionally, the tie-bar-less solution is ideally suited for the robot, which has more room to move in and out”, says Michael Feldhaus, explaining the purchasing decision.
Schott at a glance
The Schott Group has 15,000 employees worldwide and has been known for glass and everything to do with glass technologies for around 130 years. What is less known is the fact that the company also has a polymer side: every year, the company produces a total of around 10 billion ‘containers’ (syringes, cartridges, vials, ampoules) made of glass and polymer at 16 plants worldwide.
Since 2001, prefillable syringes have been produced at the Competence Centre in St. Gallen, Switzerland, from the high-tech plastic COC (cyclo olefin copolymer) under the TopPac® brand name. These are developed and produced in sizes from 1 to 50 ml and only at the St. Gallen location. Schott supplies customers worldwide with polymer syringes from Switzerland. Thanks to the highly automated systems, Switzerland is a competitive location.
Sources:
This abridged report is based on a customer report from KunststoffXtra (Sigwerb GmbH, issue May 2017, p. 8-10) by editor-in-chief Marianne Flury.